
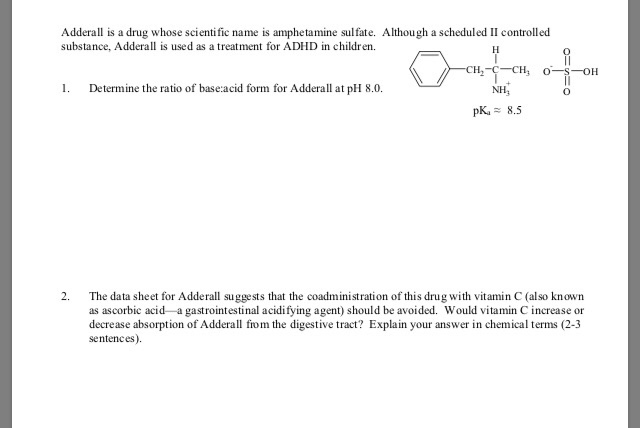
(f) No prescription for a controlled substance shall be refilled unless the original prescription provides for such refilling and unless the number of refills has been specified in said prescription. (e) All prescriptions for controlled substances shall be kept for two years by the pharmacy and shall be subject to inspection pursuant to the provisions of this chapter. (d) In regard to a controlled substance in Schedule II or III, no prescription shall be filled for more than a thirty-day supply of such substance upon any single filling provided, however, that with regard to dextro amphetamine sulphate and methyl phenidate hydrochloride, a prescription may be filled for up to a sixty-day supply of such substance upon any single filling if said substance is being used for the treatment of minimal brain dysfunction or narcolepsy provided further, that subject to regulations of the department and the board of pharmacy, prescriptions for implantable infusion pumps consisting of Schedule II or Schedule III controlled substances may be filled for a maximum of 90 days.
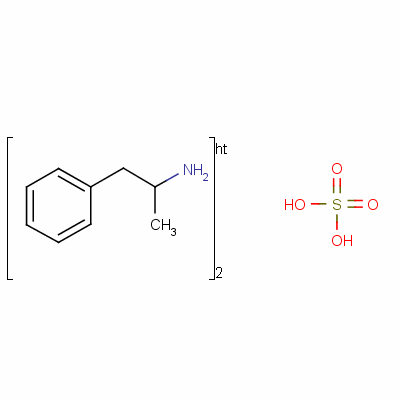
(c) The pharmacist filling a written or electronic prescription for a controlled substance in Schedule II shall endorse his own signature on the face thereof. (c) The pharmacist filling a written prescription for a controlled substance in Schedule II shall endorse his own signature on the face thereof. Written prescriptions for a controlled substance in schedule II shall be kept in a separate file. (b) A written or electronic prescription for a controlled substance in schedule II shall not be refilled.
(b) A written prescription for a controlled substance in Schedule II shall not be refilled and shall be kept in a separate file. (a) A written or electronic prescription for a controlled substance in Schedule II shall become invalid 30 days after the date of issuance. (a) A written prescription for a controlled substance in Schedule II shall become invalid 30 days after the date of issuance. Section 23: Written or electronic prescriptions requirements and restrictions


 0 kommentar(er)
0 kommentar(er)
